Oxidative stress in farm animals – the performance decreaser
What is oxidative stress in farm animals? What makes antioxidants special? What are reactive oxygen species? And what do phytogenics have to do with it? Find all the answers to your questions here…

Due to the global warming it gets hotter and hotter – not only we feel this, but also livestock animals. Due to heat stress, behavior and major physiological reactions in the organism of human and animal change:Thus, shady places are visited, sweat production or panting increases, water absorption is enhanced, and exercise is kept to a minimum. Moreover, feed intake drops, and growth performance of animals is reduced, resulting in substantial economic losses for the farmer. Do you know about oxidative stress in farm animals and its consequences? Let’s have a look:
After reading the article you will know:
- Why oxidative stress in farm animals occurs during heat stress
- What ATP has to do with energy
- What happens during lipid peroxidation
- The mode of action of antioxidants
- How selected phytogenics can offer antioxidative effects

ATP – the cells energizer
Energy can have many states: Light energy, thermal energy, electrical energy or chemical energy. Living organisms need chemical energy to maintain their vital functions. This is stored in form of different (bio)chemical compounds and can be retrieved when there is a demand for conversion into other energy forms (movement, breathing, heart rate). The most important (bio)chemical energy store of living beings is ATP (adenosine triphosphate). The Energy, released from metabolic processes, is bound in ATP. ATP consists of the nitrogen-containing base adenine, linked to ribose (5-carbon sugar). Three inorganic phosphate groups (P) are attached to ribose. If ATP reacts with water, one phosphate group is split off. ADP (adenosine diphosphate) and phosphate are formed. During this reaction, energy is released.
Cooling requires energy
Under hot and humid climate conditions, panting or sweat production to cool the organism requires energy. In animals and humans use of these cooling mechanisms is linked to an increased cellular energy expenditure. To produce sufficient amounts of energy – or adenosine triphosphate (ATP) – for essential cellular processes such as Na+-K+-ATPase activity, the capacity of mitochondria – the power plants of cells – is up-regulated. However, this upregulation of ATP-production comes along with an intensive production of reactive oxygen species.
But what are reactive oxygen species?
So-called reactive oxygen species (ROS) – also known as “free radicals” – are oxygen-containing molecules which are characterized by their high reactivity and chemical aggressiveness due to a free unpaired electron. ROS are formed in the mitochondria as a by-product of cellular respiration or by immune cells to fight bacteria or viruses.
But what are these “free radicals” doing?
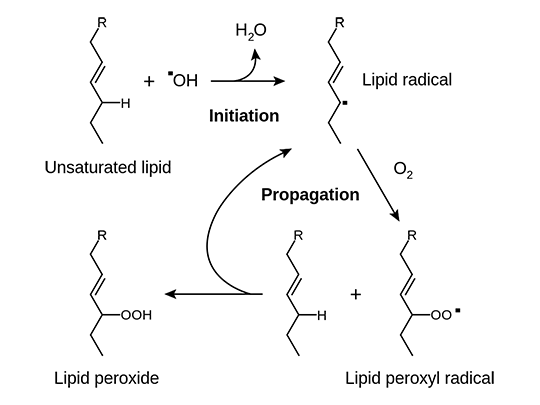
Have you ever forgot a piece of butter in the fridge, which turns rancid after a while? This rancidity of animal and vegetable derived fatty acids is caused by so-called lipid peroxidation: Let’s illustrate the activity of “free radicals” in the example of lipid peroxidation (Fig.1). Lipid peroxidation is a chain reaction and takes place in three steps: Initiation, propagation and termination. The “free radical”, in this case OH–, has an unpaired electron and therefore “seeks” for positive charge. In the initiation reaction, a nucleophilic attack occurs: the free radical OH–“steals” or splits off a positively charged H-atom from a polyunsaturated fatty acid moiety and is subsequently turned to H2O.The result is a carbon-centered lipid radical (alkyl radical), which also lacks a proton. In the propagation step, the resulting alkyl radical reacts with oxygen (O2). Consequently, a lipid peroxyl radical is formed. The resulting fatty acid peroxyl radical can now transfer its radical function by abstracting a hydrogen to other unsaturated fatty acids. Those in turn, add O2 to keep the chain reaction going or react with an antioxidant. In the latter case, the chain reaction is interrupted.
This process not only takes place in your refrigerator – but also in the body of humans and animals and means enormous stress for the structures: so-called oxidative stress.
In a healthy body, normally reductive and oxidative processes are in an equilibrium and the body has sufficient defense mechanisms to counteract excess ROS. However, energy-consuming stressors such as heat, relocation or high aerial ammonia concentrations lead to an overproduction of ROS: Thus, the body’s own protective systems, such as endogenous antioxidants, are overwhelmed and need help.
Antioxidant. What a magic word
Well, there is less magic behind antioxidants – it’s more biochemistry.
Now we come to the third phase of lipid peroxidation: termination. This means that the chain reaction of the ROS is interrupted: α- Tocopherol, the most biologically active and abundant form of vitamin E, donates its phenolic hydrogen atom to a peroxyl-radical, which in turn is converted to a hydroperoxide (Fig.2). The tocopheroxyl-radical resulting from this reaction is sufficiently stable to interrupt the chain reaction. Subsequently, the tocopheroxyl-radical is reduced, forming an ascorbate radical. The ascorbate radical is regenerated with the help of glutathione (GSH). Furthermore, the tocopheroxyl- radical reacts with another peroxyl radical leading to the formation of an inactive, non-radical product.
The lack of a sufficient quantity of antioxidants to eliminate ROS can lead to oxidative damage and inflammation. If inflammation occurs, cytokines and chemokines are brought to the site of inflammation – this – and the repair of the damaged tissue – however, again means energy expenditure for the host. As a result, more and more ROS develop, and the inflammation progresses. This is how oxidative stress in farm animals increases.

Plant derived antioxidants to the rescue!
Similar to vitamin E, numerous terpenes of aromatic plants from the Labiatae family, such as thymol or carvacrol from thyme or oregano, have proven antioxidant properties that increase the body’s resistance to oxidative stress. Selected phytogenic substances are thus able to strengthen cell defense, improve the supply of nutrients to cells and minimize damage caused by bacteria or oxidative stress in farm animals. As a result, even in periods of increased heat stress, supplementing these plant extracts to the animals feed can lead to an improved resistance to oxidative stress, which results in maintained resilience.Thus, the overall health and welfare of the animals is supported, and energy expenditure can be used for performance parameters – not for immune response.
What makes antioxidants special?
The antioxidant activity of selected phytogenics depends mainly on their molecular structure. Principally phytogenics have two different ways to unroll antioxidant effects: either directly by capturing reactive oxygen species (ROS), or indirectly by stimulating the production of the host’s antioxidant enzymes. For example, superoxidase dismutase (SOD) or glutathione peroxidase (GSH-PX) are two key antioxidant enzymes that inactivate ROS. For both it was shown, that their production can be stimulated by certain essential oils like thyme oil, turmeric or rosemary oil (Franz et al., 2010).
As an excess of ROS can lead to cell damage especially in intestinal epithelia, scavenging of those reactive molecules reduces inflammatory processes in the intestine. This results in an increased integrity of the epithelial cell wall and an improved performance via an enhanced nutrient digestibility. Placha et al. (2014) showed that thyme oil reduces the MDA content in the enterocytes and thus improves the antioxidant status and intestinal integrity. In experiments with broilers, feed additive containing essential oils, herbs, spices and saponins that stimulated the antioxidant machinery had a positive effect on intestinal morphology and significantly increased nutrient digestibility.
Finally, Yesilbag et al. (2011) demonstrated that an increased antioxidant status in broiler chicken meat prolonged the quality and shelf life of the meat by feeding diets supplemented with rosemary or its essential oils. Thus, plant derived direct or indirect antioxidants have beneficial effects on animal health and finally on product quality: they may improve the dietary nutrient value and contribute to a better stability and longer shelf-life of fat, meat and eggs.
Due to the proven positive properties – especially in terms of strengthening antioxidant capacity – phytogenic feed additives have the potential to develop into a new generation of feed additives for innovative animal nutrition and welfare. They will be a key tool in supporting livestock under heat stress conditions in the future and can therefore contribute to profitable animal production.
References upon request
You have further questions about antioxidative effects of PFAs?
We will be happy to answer them!

Anne Oberdorf
Anne has always been fascinated by the unknown, the diversity and beauty of nature. Her love for nature brought her to Delacon in 2018 after studying agricultural sciences, where she worked as Technical Communications Manager and later as Product Manager Aquaculture. Since February 2021, she has been taking a new, natural career path outside of Delacon.










